
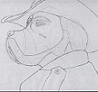
A double sided digital picture I did for the Confuzzled 2010 conbook.
The convention's topic was "Mad Scientists", so they had several topic
related, illustrated stories in their conbook (which was a very nice idea). :)
I got "Celsius vs. Fahrenheit", the two characters were chosen to be
a Siamese cat (Mr. Anders Celsius) and a Labradoodle (Mr. Daniel
Gabriel Fahrenheit).
Done with Photoshop.
Text was given to me by Confuzzled. :)
The convention's topic was "Mad Scientists", so they had several topic
related, illustrated stories in their conbook (which was a very nice idea). :)
I got "Celsius vs. Fahrenheit", the two characters were chosen to be
a Siamese cat (Mr. Anders Celsius) and a Labradoodle (Mr. Daniel
Gabriel Fahrenheit).
Done with Photoshop.
Text was given to me by Confuzzled. :)
Category Artwork (Digital) / General Furry Art
Species Dog (Other)
Size 1233 x 884px
File Size 158.9 kB
Listed in Folders
i dont think so, simply because not possible according to the laws thermodynamics^^ if you would reach absolute zero this system (a single atom or molecule for example) would be fully removed from the rest of the universe because it wouldnt be able to transfer energy anymore.
and besides, the system always keeps its zero-point energy, its impossible to extract every last bit of energy
and besides, the system always keeps its zero-point energy, its impossible to extract every last bit of energy
exactly! the entropy = S, ΔS = 0. nothing happens X3 you cant get something out of a crystal if it wont move anymore :P if you want to move it you have to use engery and thus you are not at absolute zero anymore.
but once something with energy enters the system everything would start moving again. its not like everything that enters the system will stop moving as well because the total energy in the entire system is greater than one then. the energy cant just disappear^^
but it would make sense that time stops in a system at absolute zero... if everything stops you cant distinguish between certain moments anymore. like, at all. and once you put energy back into the system it would start moving again, relatively to its last condition. so "for the molecule" time stopped :P
but once something with energy enters the system everything would start moving again. its not like everything that enters the system will stop moving as well because the total energy in the entire system is greater than one then. the energy cant just disappear^^
but it would make sense that time stops in a system at absolute zero... if everything stops you cant distinguish between certain moments anymore. like, at all. and once you put energy back into the system it would start moving again, relatively to its last condition. so "for the molecule" time stopped :P
It's been tried to reach absolute zero already and they've only gotten VERY close..about 13 degrees off or so if I can recall, and it was only for a few milliseconds. It's currently impossible to create absolute zero here on earth. But out in space, it could be possible.
no wait, i DID get the right comment!
about temps. close to absolute zero: they got VERY close to it already. like just a few degrees. superfluid helium is a good example for this^^ if you get helium barely above absolute zero it turns into a clear and very calm liquid that creeps out of any container as a very thin film until the container is empty.
http://www.youtube.com/watch?v=2Z6UJbwxBZI
about temps. close to absolute zero: they got VERY close to it already. like just a few degrees. superfluid helium is a good example for this^^ if you get helium barely above absolute zero it turns into a clear and very calm liquid that creeps out of any container as a very thin film until the container is empty.
http://www.youtube.com/watch?v=2Z6UJbwxBZI
Québec too, and it's a mess! LOL
I was totally confusing a friend in Belgium trying to explain to him that here in Québec, the ambient temperature is in C, the water temperature of a swimming pool is in F, sizes of people are in feet and inches while distances are in meters/kilometers, the weight of someone is in pound but the weight of meat at the grocery is in grams. Etc. :p It's the kind of things you need to be born in not to get confused. ;)
I was totally confusing a friend in Belgium trying to explain to him that here in Québec, the ambient temperature is in C, the water temperature of a swimming pool is in F, sizes of people are in feet and inches while distances are in meters/kilometers, the weight of someone is in pound but the weight of meat at the grocery is in grams. Etc. :p It's the kind of things you need to be born in not to get confused. ;)
I'm not quite sure I understand how you arrive at that conclusion.
"Fahrenheit added a further fixed reference point: the temperature of an equilibrium mixture of ice and salt-saturated water, which he defined as the zero point of his scale. Unfortunately, the use of three reference points added ambiguity rather than precision--the value of a degree varies by over 8% depending on which two of his reference points you choose. "
http://www.straightdope.com/columns.....erature-scales
Converting between F and C is a fairly simple procedure, there are places in the conversion that are exact and even a place that both are equal (-40C = -40F).
You could argue that 100 F is more accurate because the C conversion gives a decimal that gets rounded.
But if I told you something was 99 C exactly you'd have to go into decimals F to get an approximation.
Freezing water is an exact conversion between C and F, so is boiling. 0 C = 32 F / 100 C = 212 F
Neither C nor F is inherently more accurate than the other.
"Fahrenheit added a further fixed reference point: the temperature of an equilibrium mixture of ice and salt-saturated water, which he defined as the zero point of his scale. Unfortunately, the use of three reference points added ambiguity rather than precision--the value of a degree varies by over 8% depending on which two of his reference points you choose. "
http://www.straightdope.com/columns.....erature-scales
Converting between F and C is a fairly simple procedure, there are places in the conversion that are exact and even a place that both are equal (-40C = -40F).
You could argue that 100 F is more accurate because the C conversion gives a decimal that gets rounded.
But if I told you something was 99 C exactly you'd have to go into decimals F to get an approximation.
Freezing water is an exact conversion between C and F, so is boiling. 0 C = 32 F / 100 C = 212 F
Neither C nor F is inherently more accurate than the other.
I think I see what you are trying to say, but I still don't think that's a very stable position to take.
Because if simply having more steps is more accurate, why even bother with larger units for measuring things. Why not just simply say we have to drive 52800 feet to the store, it would be more accurate than saying 5 miles, even though they are exactly the same.
0C = 32 F = Freezing. Same thing.
100C = 212 F = Boiling. Same thing.
*shrug*
Because if simply having more steps is more accurate, why even bother with larger units for measuring things. Why not just simply say we have to drive 52800 feet to the store, it would be more accurate than saying 5 miles, even though they are exactly the same.
0C = 32 F = Freezing. Same thing.
100C = 212 F = Boiling. Same thing.
*shrug*
Neither of which is at all accurate due to rapid and considerable variances in altitude, barometric pressure, and air density. We've just grown used to them. Fahrenheit allows for a more discernible difference in local temperature readings because to change one degree in C may have a considerably different 'feel' on the observer.
100 degrees in one place could be 92 only a hundred feet away due to any number of small annoying factors.
Me, I don't care which one they use. I grew up using F, so that's what I use. Likewise with measurement... Standard v/ Metric is just flat annoying. The US should give it up and simply switch to the global majority rather than trying to force the globe to continue using ours out of sheer pride.
100 degrees in one place could be 92 only a hundred feet away due to any number of small annoying factors.
Me, I don't care which one they use. I grew up using F, so that's what I use. Likewise with measurement... Standard v/ Metric is just flat annoying. The US should give it up and simply switch to the global majority rather than trying to force the globe to continue using ours out of sheer pride.
They both have their uses. Fahrenheit is typically what people personally experience day to day (0<->100). Celsius is more manageable because it's more linear towards the elements. Kelvin's just a shifted scale of celsius so it's like his mistress or something. :3
..Now we need porn of these two double penetrating Kelvin. ;B
..Now we need porn of these two double penetrating Kelvin. ;B
Its a little more than just simply shifted...but the conversion is a simple shift.
Mathematically Kelvin is MUCH easier to work with. If you double or half the temperature of something in Kelvin you get a sane result. But try to half (or double) the temperature of something @ 0C
Its just odd math wise when you use a measurement system where a negative value is considered a normal occurrence. It would be weird to have a shoe size system normalized to the average size of a human foot, and then have people have smaller feet wearing negative sizes. Or what if we normalized height to the average, could you imagine being 5'4" and instead referring to yourself as being -4" tall ? Heck what if we had the speedometer in our cars normalized to standard highway speeds and speed limits in the city set at -30mph.
Temperature is one of the few measurement systems in everyday use that doesn't start at 0, the Kelvin(or Rankin) system attempts to address this but well we've already got 2 different systems in place and we are used to them, I can only imagine what it would be like to suddenly start thinking of a normal day 21 C (70 F) as being 294 K (530 Rankin).
"Oh its gonna be another beautiful day honey, they think the high will be 530 today"
Mathematically Kelvin is MUCH easier to work with. If you double or half the temperature of something in Kelvin you get a sane result. But try to half (or double) the temperature of something @ 0C
Its just odd math wise when you use a measurement system where a negative value is considered a normal occurrence. It would be weird to have a shoe size system normalized to the average size of a human foot, and then have people have smaller feet wearing negative sizes. Or what if we normalized height to the average, could you imagine being 5'4" and instead referring to yourself as being -4" tall ? Heck what if we had the speedometer in our cars normalized to standard highway speeds and speed limits in the city set at -30mph.
Temperature is one of the few measurement systems in everyday use that doesn't start at 0, the Kelvin(or Rankin) system attempts to address this but well we've already got 2 different systems in place and we are used to them, I can only imagine what it would be like to suddenly start thinking of a normal day 21 C (70 F) as being 294 K (530 Rankin).
"Oh its gonna be another beautiful day honey, they think the high will be 530 today"
*uses kelvin more than either of he other two* ;P
And it is a simple shift. A very simple shift. It's celsius' linearity projected back onto absolute zero instead of freezing. And on your note about negative temperatures...celsius actually hits negative before fahrenheit. ;P So if you're saying negative's an inefficient system, then you're saying celsius is more inefficient. And there's a linear conversion between C and F.
So the argument between the two is null. Just depends on what you use and is a personal preference in the end.
And it is a simple shift. A very simple shift. It's celsius' linearity projected back onto absolute zero instead of freezing. And on your note about negative temperatures...celsius actually hits negative before fahrenheit. ;P So if you're saying negative's an inefficient system, then you're saying celsius is more inefficient. And there's a linear conversion between C and F.
So the argument between the two is null. Just depends on what you use and is a personal preference in the end.
Nah not saying inefficient, just saying in some calculations it can behave oddly, and yeah as a chem major currently its been kinda fun to get the hang of using C and K for most of the calculations.
Efficiency and Accuracy aren't really part of the problem, as those are usually due to procedure or instrument limitations.
Efficiency and Accuracy aren't really part of the problem, as those are usually due to procedure or instrument limitations.
Your post get me one think: Scientists talks a lot about negative energy. Now it is still yet a theory (or I should say hypothesis instead?) but when we discover how to
make enough of this "stuff" for practical use we will have got again a negative/positive measurement in physics!
make enough of this "stuff" for practical use we will have got again a negative/positive measurement in physics!
I relate to the old standard units better, but have to use both Metric and standard at work. On the old English bikes, I sometimes have to use Imperial units for volume, and have to have a third set of wrenches (whitworth). Don't even get me started on the old pre-metric English thread patterns :grrr:
I wish we would settle on just one system, it'd be for the best, really.
I wish we would settle on just one system, it'd be for the best, really.
Still wish the US would switch to metric, I mean I live in the US and I prefer the metric system because it is so much easier but the only problem is that everyone else here seems to think otherwise and I am forced to use standard much more often that I can never get a feel of how long/heavy/etc. something is in metric.
Being an American, I really do have to say that both the metric system and the Celsuis temp system makes a lot more sense really, especially on a scientific scale. Also my dad's been agreeing because he now has a job where metric system is a lot more useful and he has to work with European folks a lot too.
Good that scientists usually use Kelvins... There are no arguments between them at least. Although Kelvins are just shifted Celsius degrees, so it's basically the same. I could never get the Fahrenheit's sense. Celsius scale is pretty natural, and very convenient for everyday use. Well... it's almost the same as with inches, feet, yards... None of them are based on the powers of 10. This makes recalculating values even harder... And something like pressure... pouds per square inch XD This makes me laugh really hard :D
I like that, "the more logical [...] metric system." I sure don't know why the imperial system is so popular in the US but its just that, illogical... The metric system is so much easier to use, not to mention its becoming more common, even on US vehicles! I have yet to find a SAE bolt head in my Ford...
You didn't explain why Fahrenheit used the references 0, 32, and 96! I know they don't make any sense now, but back then it was essential to creating a good thermometer...way better than the Celsius scale! Glass making was VERY inexact back then. Making a glass tube always created regions of thick and thin inner diameters throughout the length, so every single thermometer had to be calibrated by a chemist when the received it by marking the temperatures themselves. How did they do it? By using two very simple things every chemist had access to, and that were usually the same no matter where you go: body temperature and ice water. Now, why didn't they use a 0-100 scale? Simple! You can't divide 100 evenly by 4, 8, 16, and so on, buy you can divide them evenly into 32, which made creating a reliable scale much easier!

 FA+
FA+


























































































































Comments